Imagine a world where temperature is measured on a scale that transcends the familiar Celsius and Fahrenheit. Enter the Kelvin scale, a scientific unit that provides a precise measurement of molecular energy.
In this article, we unravel the mysteries of boiling points and explore the specific case of water. Delve into the relationship between temperature and vapor pressure, discover the impact of impurities, and learn how to calculate the boiling point of water in Kelvin.
Join us on this journey of scientific discovery and find your place in the realm of thermodynamics.
Key Takeaways
- The boiling point of water on the Kelvin scale is 373.15 K.
- Factors such as intermolecular forces and molecular size and shape influence the boiling point of water.
- The presence of dissolved substances in water can affect its boiling point, increasing it compared to pure water.
- Understanding the relationship between temperature and vapor pressure is crucial in determining the boiling point of a substance.
The Kelvin Scale and Temperature Conversion
The Kelvin scale, which is widely used in scientific research and measurement, allows for precise temperature conversion through the use of a subordinating conjunction.
Unlike the Celsius and Fahrenheit scales, the Kelvin scale is an absolute temperature scale that starts from absolute zero, the point at which all molecular motion ceases. This makes it particularly useful in scientific calculations and experiments where accurate temperature measurements are crucial.
The conversion from Celsius to Kelvin is relatively straightforward, as Kelvin is simply the Celsius temperature plus 273.15. This conversion allows for easy comparison and analysis of temperature data across different scales.
In scientific research, the use of the Kelvin scale ensures consistency and accuracy in temperature measurements, providing a standardized system for temperature evaluation and analysis.
Understanding Molecular Energy and Boiling Points
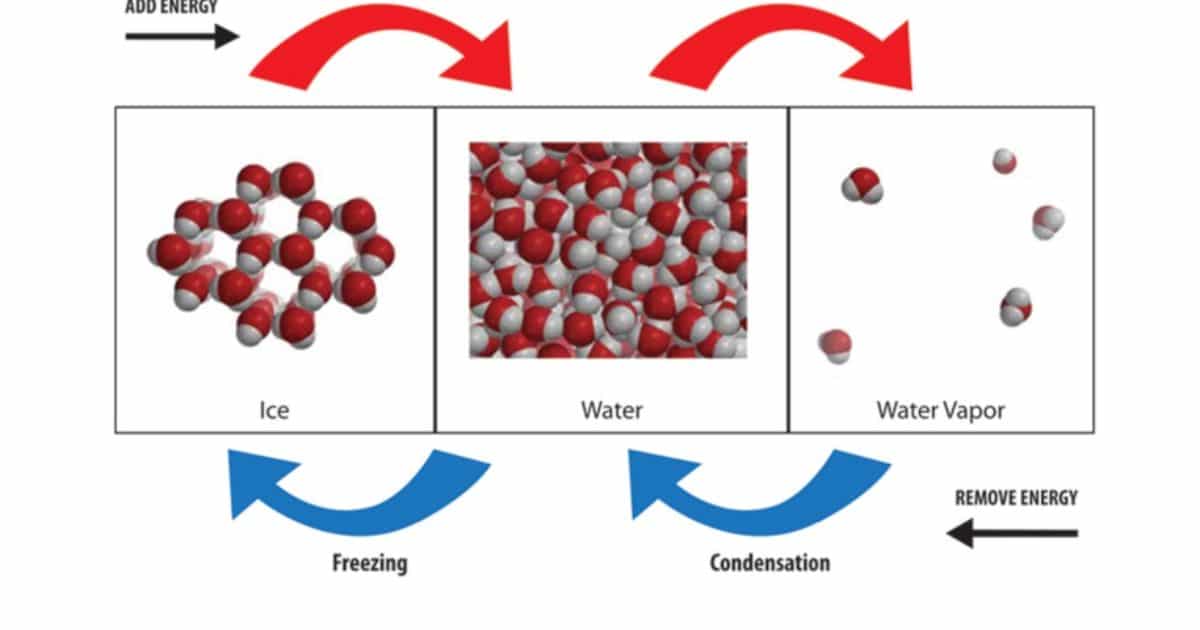
To further explore the topic of molecular energy and boiling points, it is important to delve into the specific factors that influence the boiling point of water in Kelvin. Understanding the molecular energy and its relationship to boiling points can provide valuable insights into the behavior of substances at different temperatures.
One key factor that influences the boiling point of water is the strength and type of intermolecular forces present in the substance. These forces determine the energy required to break the bonds between molecules and transition from a liquid to a gas phase. The molecular size and shape of water molecules also play a role in determining the boiling point. The table below summarizes the factors influencing the boiling point of water in Kelvin.
| Factors Influencing Boiling Point of Water in Kelvin |
|---|
| Intermolecular forces |
| Molecular size and shape |
| Atmospheric pressure |
| Impurities |
Understanding these factors can deepen our understanding of the boiling point of water and how it can be affected by different conditions. With this knowledge, we can now explore the relationship between temperature and vapor pressure, which further explains the behavior of water at different boiling points. For instance, one common experiment involves observing what happens when you put boiling water in a glass, shedding light on how the physical properties of the container may influence the boiling process.
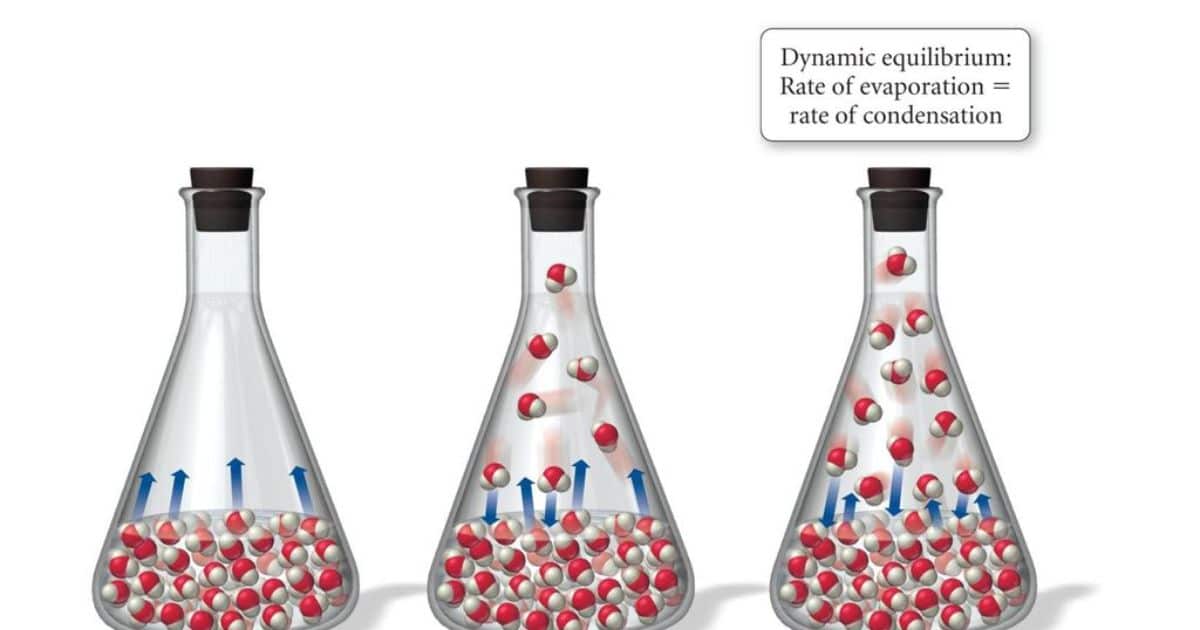
The Relationship Between Temperature and Vapor Pressure
Understanding the relationship between temperature and vapor pressure is crucial for comprehending the behavior of water at different boiling points. The vapor pressure of a substance is the pressure exerted by its vapor when it is in equilibrium with its liquid phase. Here are three key points to consider:
- As temperature increases, the vapor pressure of a substance also increases. This is due to the increased kinetic energy of the molecules, which leads to more molecules escaping from the liquid phase and entering the vapor phase.
- The relationship between temperature and vapor pressure is exponential. This means that a small increase in temperature can lead to a significant increase in vapor pressure.
- The boiling point of a substance is reached when its vapor pressure equals the external pressure. At this point, the liquid rapidly changes to a gas, resulting in the formation of bubbles.
Boiling Points of Different Chemical Elements
Different chemical elements have varying boiling points. The boiling point of an element is the temperature at which it changes from a liquid to a gas. It is an important physical property that helps determine the state of matter in which an element exists under specific conditions.
The boiling point is influenced by factors such as intermolecular forces, atomic size, and molecular structure. For example, elements with stronger intermolecular forces, such as hydrogen bonding, tend to have higher boiling points.
The boiling points of different chemical elements can range from very low temperatures, such as -252.87 degrees Celsius for helium, to extremely high temperatures, such as 5,555 degrees Celsius for tungsten.
Understanding the boiling points of different elements is crucial in various scientific and industrial applications, including distillation, chemical reactions, and material synthesis.
The Boiling Point as a Reference Property
Boiling point serves as a fundamental reference property for characterizing the behavior of substances undergoing phase changes. It is a crucial parameter used in various scientific fields, such as chemistry, physics, and engineering.
Here are three key reasons why the boiling point is an essential reference property:
- Identification: The boiling point helps identify substances. Different compounds have unique boiling points, allowing scientists to determine the composition of unknown substances by comparing their boiling points with known compounds.
- Purity Assessment: The boiling point can assess the purity of a substance. Impurities tend to affect the boiling point, causing variations from the expected value. By measuring the boiling point, scientists can determine the level of purity or the presence of impurities in a sample.
- Process Control: The boiling point is used to control industrial processes. It helps determine the optimal temperature for boiling reactions, distillation, and separation processes, ensuring efficient and precise operations.
Understanding the boiling point as a reference property enhances our ability to study and manipulate substances in various scientific and industrial applications.
Impact of Impurities and Mixtures on Boiling Points
Impurities and mixtures can significantly influence the boiling points of substances, thereby impacting their behavior and properties. When a pure substance is mixed with impurities, the boiling point of the resulting mixture can be raised or lowered compared to the pure substance. This phenomenon occurs because impurities disrupt the intermolecular forces between the molecules of the substance, affecting the energy required for vaporization.
The table below illustrates the impact of different impurities and mixtures on boiling points:
| Impurity/Mixture | Boiling Point Change |
|---|---|
| Salt | Increased |
| Sugar | Increased |
| Alcohol | Decreased |
| Acid | Increased |
| Metal | Increased |
Understanding the impact of impurities and mixtures on boiling points is crucial in various industries, such as pharmaceuticals and chemical engineering, where precise control of boiling points is essential for product quality and process efficiency. By studying these effects, scientists and engineers can optimize processes and enhance the desired properties of substances.
Table: Boiling Points of Water at Different Pressures
The boiling point of water varies depending on the pressure it is subjected to. Understanding this relationship is crucial for various scientific and industrial applications.
Here is a table showcasing the boiling points of water at different pressures:
- At sea level (standard atmospheric pressure of 1 atmosphere), the boiling point of water is 100 degrees Celsius or 212 degrees Fahrenheit.
- As pressure decreases, such as at higher altitudes, the boiling point of water decreases as well. For example, at an altitude of 2,000 meters, the boiling point of water drops to approximately 94 degrees Celsius or 201 degrees Fahrenheit.
- Conversely, as pressure increases, the boiling point of water increases. At a higher pressure, water requires more energy to overcome the increased pressure and reach its boiling point.
This table serves as a useful reference for scientists, engineers, and individuals working with water under varying pressure conditions.
Factors Affecting Boiling Point Elevation
An important factor affecting the elevation of the boiling point is the presence of dissolved substances in the water. When a solute is added to water, it disrupts the intermolecular forces between water molecules, making it more difficult for them to escape into the gas phase. This results in an increase in the boiling point of the solution compared to pure water.
The degree of boiling point elevation depends on the concentration of the solute. This phenomenon is commonly observed when cooking pasta or adding salt to boiling water.
How to Calculate the Boiling Point of Water in Kelvin
Calculation of the boiling point of water in Kelvin requires understanding the relationship between temperature and pressure. Below are the steps to calculate the boiling point of water in Kelvin:
- Convert the temperature to Kelvin: Kelvin is an absolute temperature scale where 0 Kelvin is equivalent to absolute zero (-273.15 degrees Celsius). To convert Celsius to Kelvin, simply add 273.15 to the Celsius temperature.
- Determine the pressure: The boiling point of water is influenced by the pressure exerted on it. Standard atmospheric pressure at sea level is 1 atmosphere (atm), which is equivalent to 101.325 kilopascals (kPa). If the pressure is different, adjust accordingly.
- Use the boiling point equation: The boiling point of water increases with increasing pressure. The equation to calculate the boiling point is:
Boiling Point (Kelvin) = Boiling Point at Standard Pressure (Kelvin) + Pressure Increase (Kelvin)
Conclusion
In conclusion, the boiling point of water in Kelvin is 373.15 K. This temperature conversion is based on the Kelvin scale, which is used in scientific and technical fields to measure temperature. Understanding the relationship between temperature and vapor pressure is crucial in determining the boiling point of water and other chemical elements.
The boiling point of water can vary depending on external factors such as pressure and impurities. By accurately calculating the boiling point, scientists can better understand the behavior of water and its properties.











